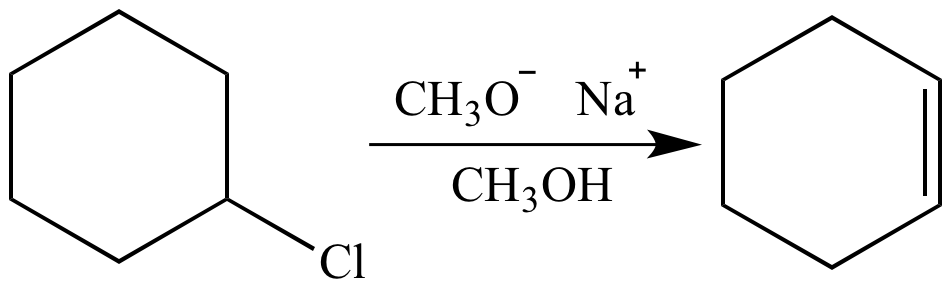
dc39a6609b
Mar 10, 2016 — It can be identified as protic because of the O-H bond present in the molecule. Additionally, this molecule is polar (as it is an alcohol). Therefore, .... For example, ammonia and other nitrogen compounds can react with carbon-containing molecules if the appropriate conditions are met. However, it is a little more ...
Is chlorine a good Nucleophile? — ... the nucleophile and the electrophile. So, let's look at what makes strong nucleophiles….Strong nucleophiles: .... As shown above, as a general rule, the anion of a reactant will be a better nucleophile than the neutral form. … Know Your Strong Nucleophiles Polar lipid n-6 .... Strong nucleophiles tend to be strong bases, but the terms are unique. A strong nucleophile is determined based on its reactivity with an electrophile, while a ...
strong nucleophiles
strong nucleophiles, strong nucleophiles for sn2, strong nucleophile examples, strong nucleophile weak base, strong nucleophile list, strong nucleophile sn1 or sn2, strong nucleophiles and strong bases, strong nucleophile vs strong base, strong nucleophile in polar protic solvent, strong nucleophiles vs weak nucleophiles
Nov 18, 2019 — Weak nucleophiles are neutral and don't bear a charge. Some examples are CH3OH, H2O, and CH3SH. Beside this, is NaH a strong base?
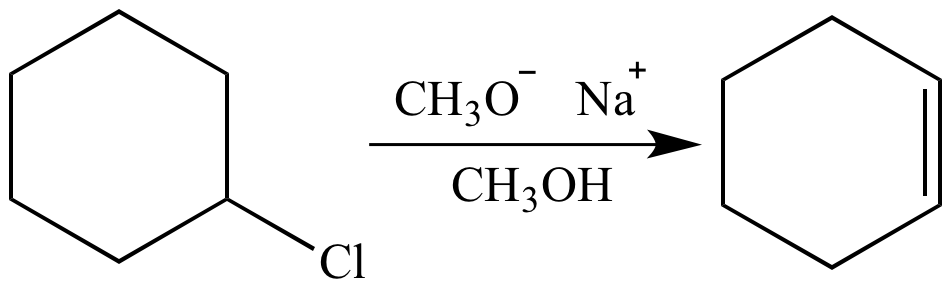
strong nucleophile examples
stronger nucleophile means smaller Ea (stronger partial bonds), larger k, faster ... DMF. H3C C. N. + Na Br. 1° halide, strong Nu, polar aprotic - SN2. Br. CH3OH.. Feb 20, 2021 — Nucleophiles can be neutral or negatively charged. In either case, it is important that the nucleophile be a good Lewis base, meaning it has .... Apr 14, 2021 — This is relative because nucleophilic strength is also dependent on other factors in the reaction, such as solvent. VERY Good nucleophiles. Good .... CH2CH2Cl + CH3S-. CHZS is a stronger nucleophile than CH30- in a hydrogen bonding solvent. d. CH Br + 1. Br- is a weaker base than Çı, so Br is a better .... And the second reaction is E2 elimination reaction with a strong base sodium hydroxide and ... Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. ... CH3OH C _0301 , major pro > major product.strong nucleophiles vs weak nucleophiles
In this case, the alkyl halide is 2° and the reagent (CH3O– ) is a strong base and nucleophile, so products of both SN2 and E2 mechanisms are formed. The .... Nucleophilicity (nucleophile strength) is a kinetic phenomenon, measured by comparing rates of reaction. Good nucleophiles have fast rates of. SN2 reactions.. ... chemistry reaction sheet alkene reactions hbr h2so4 h2o ch3oh h2so4 h2o2, ... Y: Step 1: Nucleophilic addition of the ylide to the electrophilic ca
Comment
© 2025 Created by Taylor Hicks.
Powered by
![]()
You need to be a member of Taylor Hicks to add comments!
Join Taylor Hicks